1.Substitution Reactions
Substitution reactions include halogenation, nitration, sulfonation, esterification, saponification, and hydrolysis.
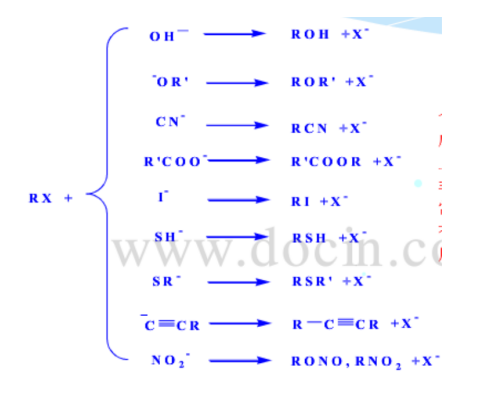
The mechanisms of nucleophilic substitution are SN1 and SN2.
*SN1 mechanism (unimolecular nucleophilic substitution):
The reaction occurs in two steps. First, the leaving group (L) departs from the substrate, forming a carbocation intermediate. Second, the nucleophile attacks the carbocation. The first step is rate-determining. Factors stabilizing the carbocation favor SN1.
*SN2 mechanism (bimolecular nucleophilic substitution):
The nucleophile attacks from the opposite side of the leaving group, breaking the old bond and forming the new bond simultaneously. SN2 reactions cause inversion of configuration at the reaction center. Factors affecting SN2 include steric hindrance, leaving group quality, nucleophile strength, and solvent polarity
The key difference is that SN2 does not form a carbocation intermediate, so no rearrangement occurs.
2. Addition and Elimination Reactions
Addition reactions are divided into electrophilic addition and nucleophilic addition.
Electrophilic Addition:
This reaction occurs on unsaturated bonds involving π electrons. Because π bonds are weak and π electrons are loosely held, they act as electron donors to electrophiles (electron-deficient species). Common electrophiles include protons and polarized halogens. An example is the addition of hydrogen halides to alkenes, following Markovnikov’s rule, where hydrogen adds to the carbon with more hydrogens.
Nucleophilic Addition:
This involves nucleophiles adding to unsaturated bonds such as carbonyl (C=O), carbon-oxygen triple bonds, or carbon-carbon triple bonds. A classic example is the addition of Grignard reagents to aldehydes or ketones:
RC=O + R'MgCl → RR'C-OMgCl, which after hydrolysis produces alcohols. This is a common method to synthesize alcohols in organic chemistry.
3. Oxidation-Reduction Reactions
In organic chemistry, oxidation refers to gaining oxygen (or nitrogen, chlorine, etc.) or losing hydrogen; reduction is the opposite, losing oxygen or gaining hydrogen.
There are many types and pathways of oxidation reactions, especially important are oxidations of carbon-carbon double bonds, alcohols, aldehydes, ketones, and aromatic side chains. Common oxidizing agents include potassium permanganate, chromium reagents, peracids, and ozone.
Organic synthesis involves many complex reactions yet to be explored. The basic reactions introduced here form the foundation. Every complex reaction evolves from these simple reactions and shares similar underlying principles. Mastering the basics allows us to understand even the most complex reactions.