With the rapid development of industry, CO₂ emissions continue to increase year by year, making the research on novel and efficient CO₂ capture and storage technologies and materials a focus of attention. Liquid-phase chemical absorption technology, which is easy to operate continuously and scale up, is considered the most mature CO₂ capture technology. Among liquid absorbents, amine-based aqueous solutions have been used for industrial CO₂ capture, but they suffer from high regeneration energy consumption, strong equipment corrosion, and high toxicity. Solid-phase adsorption is another promising method for CO₂ removal, using materials such as metal–organic frameworks (MOFs), zeolites, and porous carbons. However, most of these adsorbents are affected by water or suffer from physical aging, making continuous operation difficult. Therefore, many researchers are committed to developing novel adsorption materials with low energy consumption, good fluidity, and suitability for continuous industrial processes.
Porous ionic liquids (PILs) have emerged as a new type of liquid adsorbent. Among them, PILs based on ZIF-8 as a microporous framework have become representative materials due to their excellent designability and outstanding adsorption performance. Some studies have developed a novel type of ZIF-8 PILs composed of conventional ILs and amine-functionalized ZIF-8 for CO₂ capture. However, these materials exhibited low CO₂ absorption capacity due to the lack of strong synergy between the IL and the ZIF-8 framework. Imidazolium-based ILs with primary amine functionalization and bis(trifluoromethylsulfonyl)imide ([Amim][NTf₂]) as the anion have been widely studied for CO₂ capture owing to the highly efficient synergistic effect of the functionalized amine groups and their low viscosity. Nevertheless, the direct interaction between the IL and ZIF-8, and how these interactions affect CO₂ absorption performance, remain to be thoroughly investigated.
Based on this, the research team led by Wang Qiang from Shandong University of Science and Technology proposed a dual-amine functionalization strategy. They dispersed MEA@ZIF-8, a microporous framework, into [Amim][NTf₂] and successfully developed the MEA@ZIF-8-Amim-PIL material. This material enables highly efficient CO₂ capture and cyclic regeneration under low-pressure conditions, providing an innovative solution for industrial carbon capture technology.
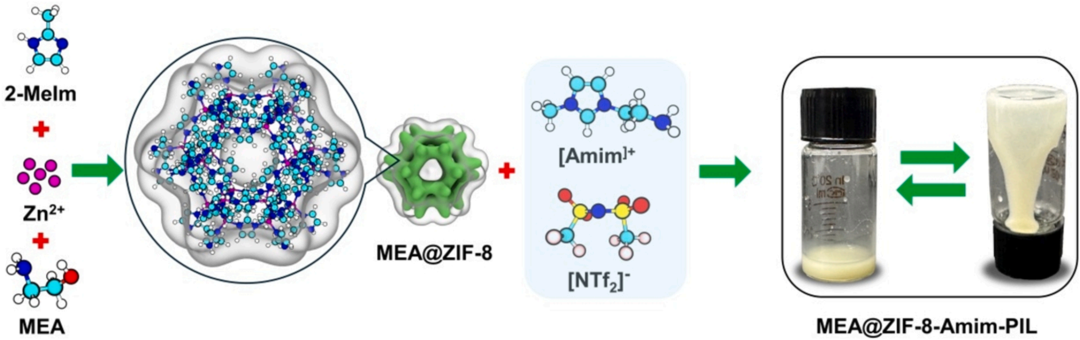
Figure 1. Schematic diagram of the synthesis process of MEA@ZIF-8-Amim-PIL.
Firstly, the research team synthesized the porous framework material MEA@ZIF-8 via an in-situ doping strategy. Using zinc ions as metal nodes and 2-methylimidazole as the ligand, the ZIF-8 framework was constructed, while monoethanolamine (MEA) was introduced during the synthesis process to anchor its -NH₂ groups onto the ZIF-8 surface via coordination bonds. SEM characterization results showed that MEA@ZIF-8 maintained the regular dodecahedral morphology of ZIF-8. The specific surface area of the material was measured to be 1311 m²/g, which is slightly lower than that of pristine ZIF-8 (1718 m²/g), confirming the successful incorporation of MEA into the pores.
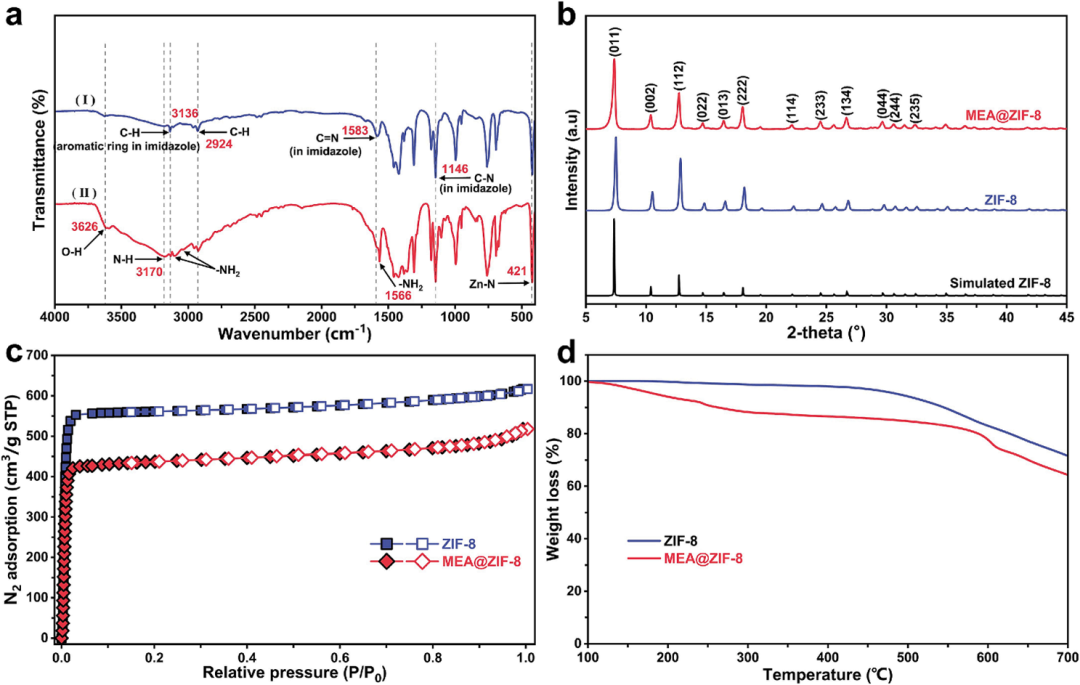
Figure 2. Synthesis and Structural Characterization of MEA@ZIF-8
Subsequently, the research team selected the amine-functionalized ionic liquid AmimNTf₂, which contains primary amine groups (-NH₂), as the dispersion medium to provide chemical adsorption sites. The NTf₂⁻ anion exhibits low viscosity (89 mPa·s at 25 °C), facilitating CO₂ diffusion. MEA@ZIF-8 was uniformly dispersed in AmimNTf₂ via a solvent mixing method, resulting in the formation of a stable MEA@ZIF-8-Amim-PIL. Molecular dynamics simulations revealed that the pore size of ZIF-8 (3.4 Å) is significantly smaller than the molecular dimensions of the IL ions (Amim⁺: 9.6 Å; NTf₂⁻: 9.4 Å), ensuring that the pores remain unblocked.
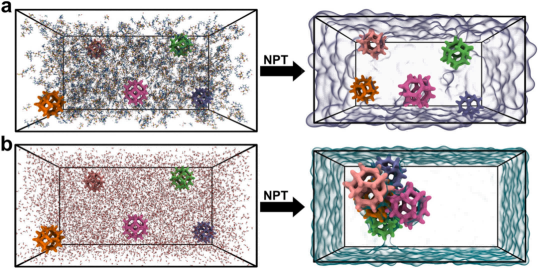
Figure 3. Molecular Dynamics Simulation Results
Subsequently, the research team evaluated the CO₂ absorption performance of the material. Under conditions of 25 °C and 1 bar, the CO₂ absorption capacity of MEA@ZIF-8-Amim-PIL reached 94.3 mg/g, which is nearly five times higher than that of pristine ZIF-8 (27.36 mg/g) and significantly exceeds that of the pure ionic liquid AmimNTf₂ (57.9 mg/g). Cycling tests demonstrated that the material retained over 98% of its absorption capacity after five adsorption-desorption cycles, with complete regeneration achievable at 80 °C. Comparative experiments revealed that the non-functionalized ZIF-8-Amim-PIL exhibited an absorption capacity of 82.4 mg/g, confirming that the dual-amine synergistic effect contributes an additional 14.5% capacity enhancement. FT-IR spectra showed the emergence of a characteristic carbamate (-NHCOO⁻) peak at 1573 cm⁻¹ after CO₂ adsorption, which disappeared following desorption, indicating a reversible chemical reaction between -NH₂ and CO₂.
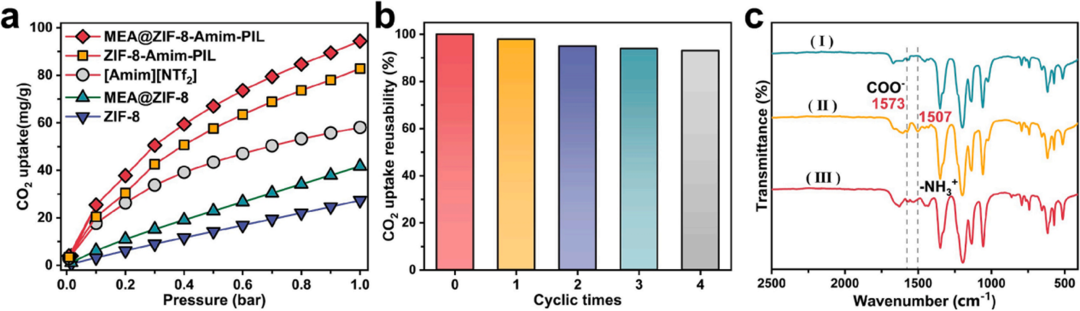
Figure 4. Comparison of CO₂ Absorption Performance and Chemical Bond Changes After CO₂ Adsorption
Finally, to elucidate the mechanism behind the performance enhancement, the research team conducted an in-depth investigation combining spectroscopic analysis and theoretical calculations. DFT calculations revealed that the -NH₂ groups from MEA on the ZIF-8 surface and the -NH₂ groups in Amim⁺ form dual adsorption sites, with CO₂ adsorption energies of -1.8 eV and -2.1 eV, respectively, significantly higher than that of the single amine system (-1.2 eV). Electrostatic potential (ESP) analysis uncovered a charge density difference (Δρ = 1.2 eV) at the interface between ZIF-8 and AmimNTf₂, which can induce the enrichment of CO₂ within the pores.
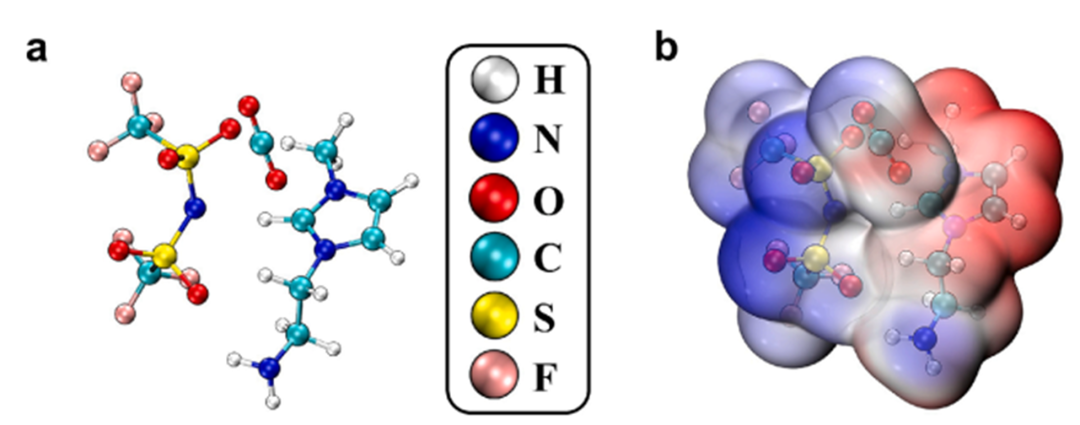
Figure 5. Interaction Sites Between AmimNTf₂ and CO₂
In summary, this study breaks through the limitation of conventional PILs that rely on high-pressure adsorption. Through a synergistic strategy combining dual-amine functionalization and pore engineering, efficient CO₂ capture is achieved under low-pressure conditions (1 bar), making it suitable for direct treatment of flue gas from coal-fired power plants (0.1–0.2 bar). Compared to commercial amine solutions, the regeneration energy consumption is reduced by 40%, without issues of volatility or corrosion. This work contributes to understanding the CO₂ absorption mechanism of PILs and facilitates the development of high-performance functional PIL materials for industrial CO₂ capture.
Author of the Original Text:Pingping Zhao, Zhihao Li, Baikang Dong, Lu Zhang, Xin Su, Qiang Wang, Di Liu, Dongmei Xu, and Jun Gao
Guangzhou Lingo Scientific Co., Ltd. — Delivering professional solutions and technical support for the global chemical industry. Our expert team is always ready for your technical inquiries! EMAIL:sales@lingochem.com