With the advancement of technology and the prosperity of the information industry, germanium (Ge) has become increasingly prominent as a critical strategic metal. Ge is widely used in electronics and semiconductors, the fiber optics industry, infrared optics, and other fields. In Europe and the United States, approximately 7% of Ge is used in semiconductors, 20% in fiber optics, and 55% in infrared optics. Global demand for Ge continues to rise, and the fiber optics market is expected to reach $372 million in the future, further driving the growth in Ge demand. However, Ge resources are scarce and unevenly distributed, with limited known global reserves. Currently, Ge recovered from minerals such as coal gangue and zinc concentrate accounts for only about 3% of the total demand, and traditional extraction methods can no longer meet the growing demand. Waste electronic products, such as discarded fiber optics, contain a certain amount of Ge, making their recycling of significant strategic importance and economic value. Existing recycling technologies have many limitations: for instance, pyrometallurgy is energy-intensive and yields low-purity products; solvent extraction in hydrometallurgy suffers from issues such as extractant loss and long extraction times; and some emerging recycling technologies, like vacuum phosphoric acid reduction, face challenges such as high costs, complex processes, and high energy consumption in large-scale industrial applications.
Against this backdrop, ionic liquids (ILs), as a novel green solvent, have garnered significant attention in the field of metal extraction due to their unique properties, such as good thermal stability, low volatility, non-flammability, high thermal stability, and the inability to release H⁺ in water. Phosphazene-based ionic liquids (PILs), such as Cyphos ILs, have demonstrated excellent performance in extracting various metal ions and have been widely used to extract multiple metal ions, including Ge, from synthetic solutions and leachates.
Based on the above considerations, a team led by Amit Kumar from Nanjing University of Information Science and Technology, Soniya Dhiman from the Indian Institute of Technology Delhi, and Himanshu Gupta from IFTM University Moradabad in India proposed a method for recovering Ge from waste fiber optics. The study marks the first use of Cyphos IL 104 (dissolved in toluene) to facilitate the recovery of Ge from such fiber optics.
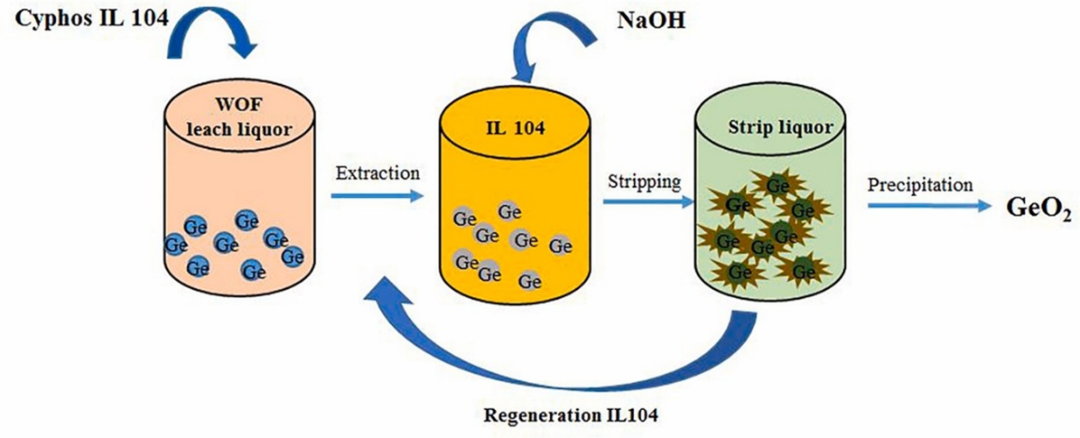
Figure 1. Recovery of Ge from waste optical fiber using phosphazene ionic liquid
The team separated the optical fiber portion from the waste optical fibers, ground it into powder, and dried it at 70°C for 24 hours. Subsequently, 5 grams of the ground waste optical fiber powder were heated with 50 mL of 7 mol/L hydrochloric acid at 80°C for 4 hours, and the leachate was obtained after filtration. The leachate contained metal ions such as Ge, Fe, Si, Ca, and Mg. The metal content in the leachate was analyzed using microwave plasma atomic emission spectroscopy (MP-AES). The leaching efficiencies were found to be 97.4% for Ge, 99.2% for Fe, 43.5% for Si, 97.8% for Ca, and 96.9% for Mg.
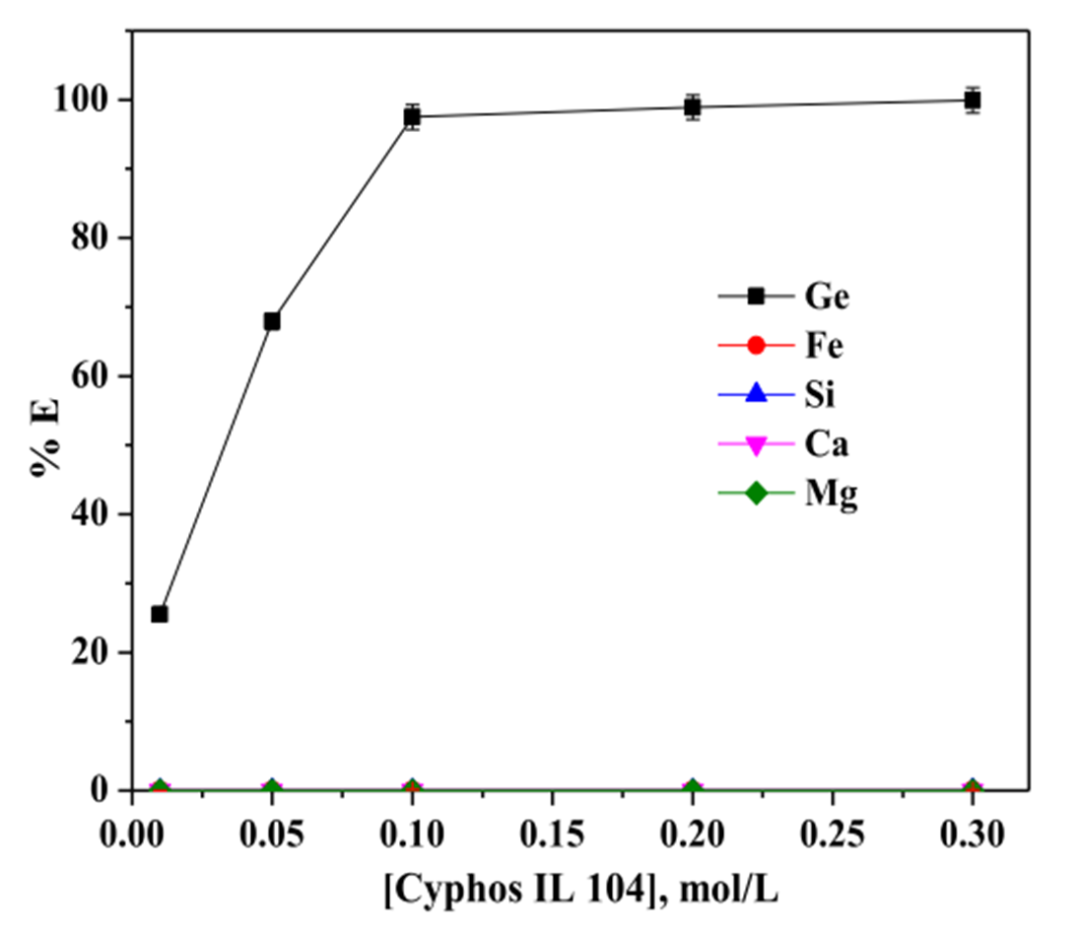
Figure 2. Optimization of Cyphos IL 104 concentration for germanium extraction from leachate.
Subsequently, the team equilibrated different concentrations of Cyphos IL 104 (0.01–0.3 mol/L) with the leachate at an organic-to-aqueous phase ratio (O/A) of 1:1 to determine the optimal extraction conditions. Experimental results demonstrated that the extraction rate of germanium reached quantitative levels when the concentration of Cyphos IL 104 was 0.3 mol/L. At a concentration of 0.1 mol/L, the extraction efficiency of germanium remained relatively high, while the co-extraction of other metals was negligible.
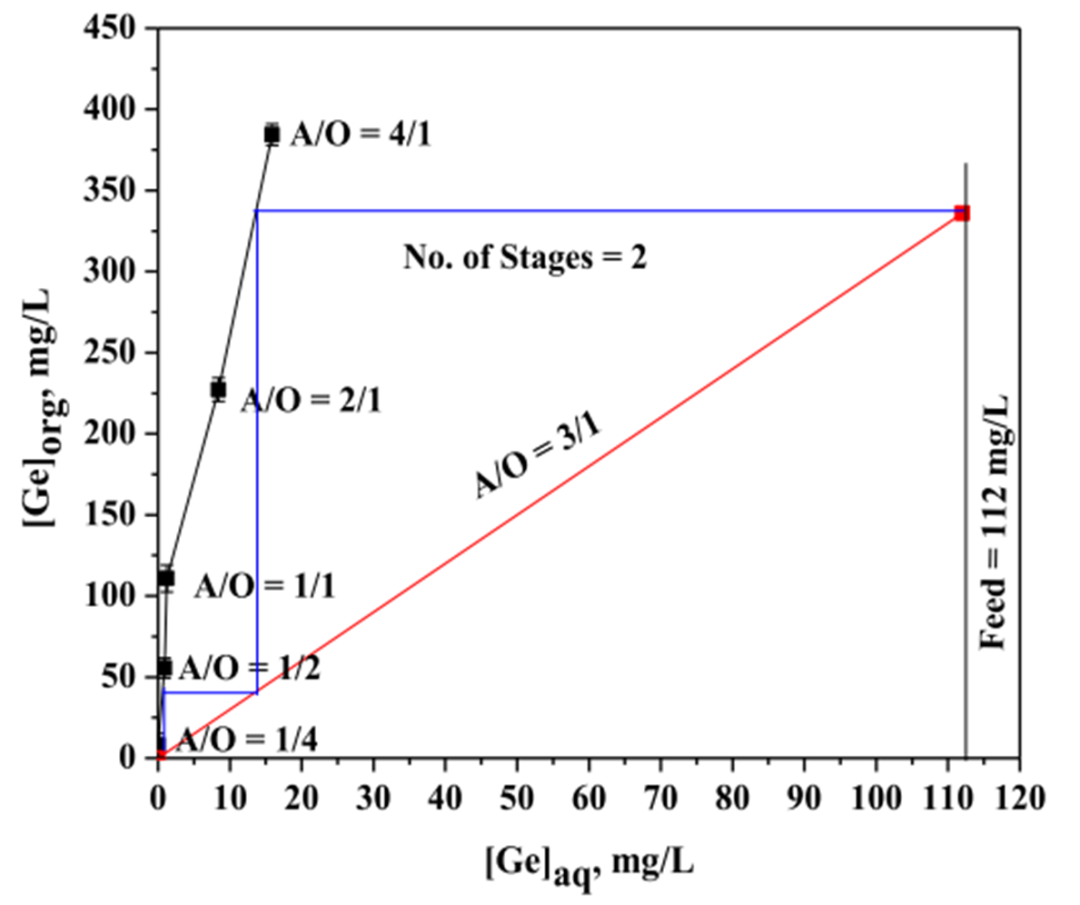
Figure 3. McCabe-Thiele diagram for germanium extraction.
Subsequently, the team mixed aqueous phase (Ge-containing leachate) and organic phase (Cyphos IL 104 in toluene solution) at varying volume ratios and agitated the mixtures in a mechanical shaker for 5 minutes to achieve equilibrium. The aqueous-to-organic phase ratios (A/O) were set at 1/4, 1/2, 1/1, 2/1, and 4/1, respectively. The McCabe-Thiele diagram was constructed by measuring the germanium concentration in the aqueous phase and calculating its concentration in the organic phase.
The diagram indicates that at an A/O ratio of 3/1, the extraction curve demonstrates that complete extraction of germanium can be achieved in just two theoretical stages, with the germanium concentration in the organic phase reaching approximately 325 mg/L. The saturation extraction curve further confirms that under an A/O ratio of 3/1, the organic phase efficiently extracts germanium from the aqueous phase with a low number of theoretical stages required.
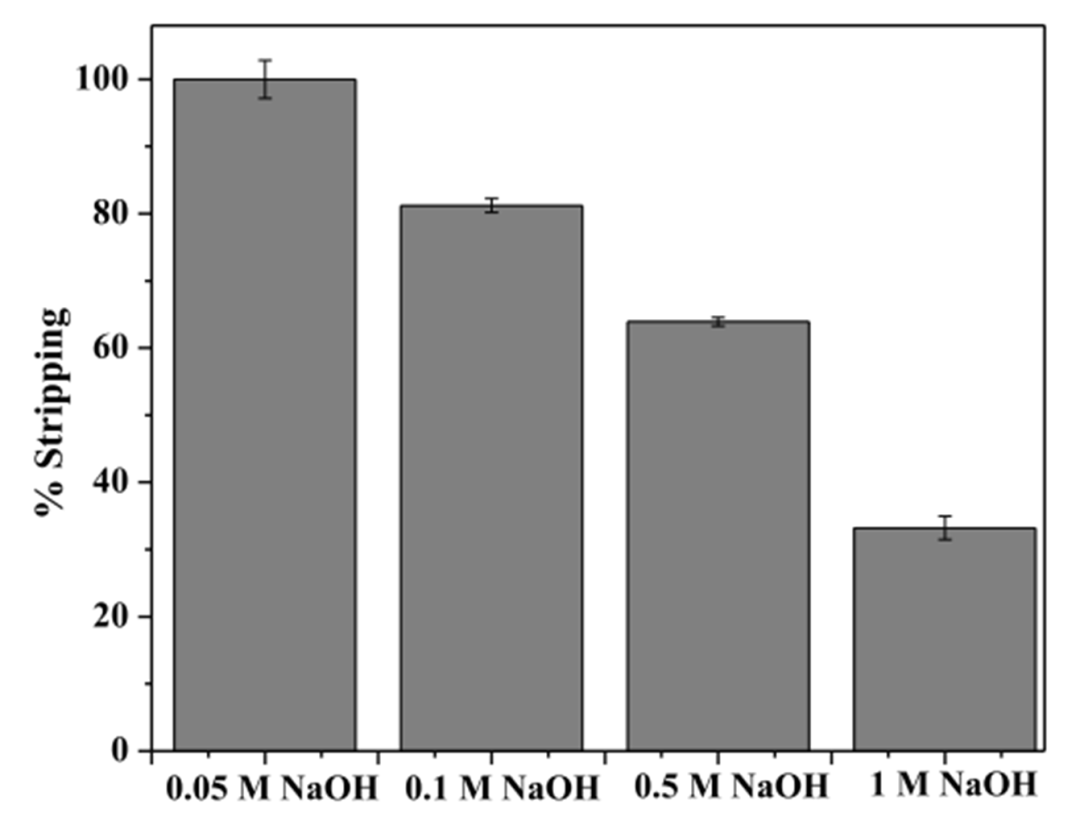
Figure 4. Effect of sodium hydroxide concentration on germanium stripping from loaded organic phase.
Next, using a two-stage counter-current extraction process with 0.1 mol/L Cyphos IL 104 at an A/O ratio of 3:1, the team achieved a germanium extraction efficiency of 99.9%. For the stripping process, experiments were conducted using NaOH solutions at varying concentrations to strip germanium from the loaded organic phase. The results identified 0.05 mol/L NaOH solution as the optimal stripping agent, requiring three stripping stages for effective recovery.
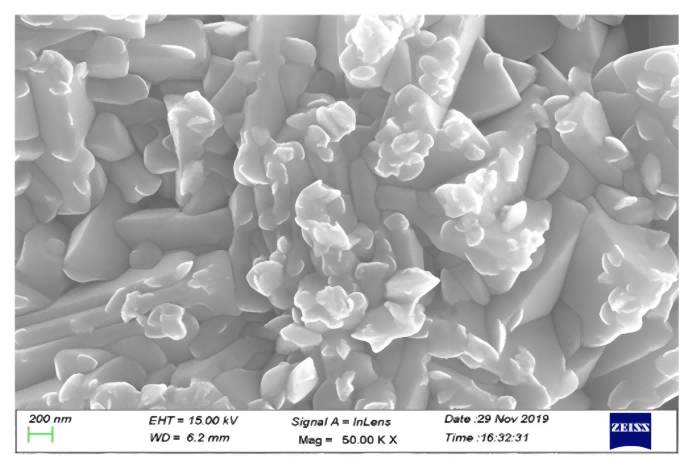
Figure 5. FE-SEM image of synthesized GeO₂.
Finally, the team precipitated Ge(OH)₄ from the stripped solution by pH adjustment, followed by roasting at 350 °C to obtain GeO₂. In summary, this study provides an efficient method for recovering germanium from waste optical fibers and synthesizing high-purity GeO₂. The approach demonstrates environmental friendliness, high efficiency, and strong selectivity, offering a new pathway for germanium recycling.
Original Author(s):Amit Kumar, Soniya Dhiman, Rupesh Kumar, and Himanshu Gupta
Guangzhou Lingo Scientific Co., Ltd. — Delivering professional solutions and technical support for the global chemical industry. Our expert team is always ready for your technical inquiries! EMAIL:sales@lingochem.com