English Name: Lithium Tri-t-butoxyaluminum Hydride
Molecular Formula: C₁₂H₂₈AlLiO₃
Molecular Weight: 254.32
CAS Number: 17476-04-9
Abbreviations and Aliases: LTBA
Physical Properties: Solid powder, mp 300–319 °C, sublimes at 280 °C/2 mmHg (266.6 Pa). Soluble in tetrahydrofuran, dimethyl ether, diglyme (diethylene glycol dimethyl ether), and diethyl ether.
Preparation and Availability: Commercially available from major international chemical suppliers as standardized solutions in various solvents and concentrations, such as 0.5 M or 1.0 M solutions in diglyme or tetrahydrofuran. It can also be prepared by reacting 3.0 M tert-butanol with 1.0 M AlLiH₄.
Safety Notes: Both the solid and solutions are corrosive and flammable. Must be stored and handled under inert conditions, protected from air and moisture.
Lithium tri-tert-butoxyaluminum hydride (LTBA) is a commonly used reducing agent i
n the laboratory. This reagent can deliver one equivalent of hydride (H⁻) in reactions, allowing for precise control over stoichiometry.
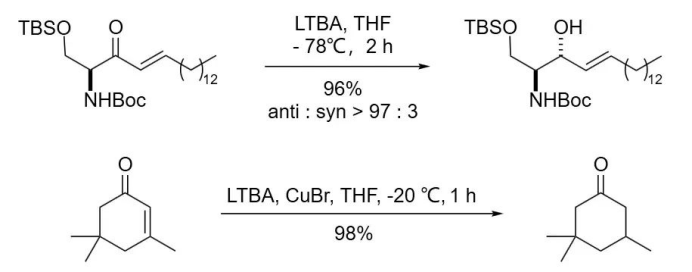
Derived from AlLiH₄, LTBA exhibits weaker reducing power than AlLiH₄ but stronger than NaBH₄. It is particularly useful for the reduction of aldehydes and ketones to their corresponding alcohols. When both aldehydes and ketones are present in the system, LTBA selectively reduces aldehydes first.

At -78 °C, unsaturated acyl chlorides can be reduced by LTBA to yield unsaturated aldehydes.
When aryl disulfides are used as substrates, thiophenols are obtained.
In the case of isocyanates, the reaction produces formamide derivatives.
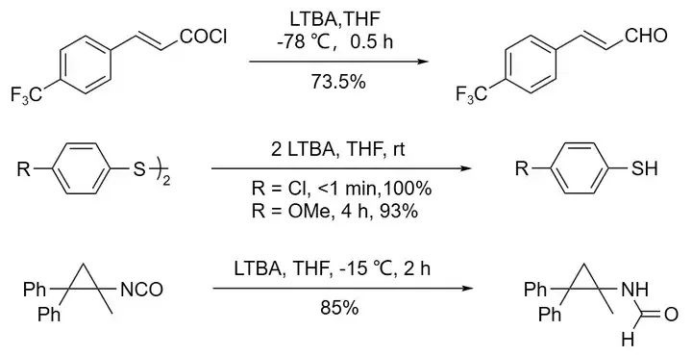
Many functional groups that can be reduced by AlLiH₄ (such as nitro, cyano, amide, imine, ether, carboxylic acid, and carboxylate esters) cannot be reduced by LTBA. Additionally, the reduction of epoxides with LTBA proceeds very slowly.
For alkyl halides, both AlLiH₄ and LTBA rapidly reduce alkyl iodides. However, LTBA reduces alkyl bromides much more slowly and fails to reduce alkyl chlorides entirely.
While ordinary carboxylate esters are generally unreactive toward LTBA, diethyl malonate can be selectively reduced to the corresponding monoester.
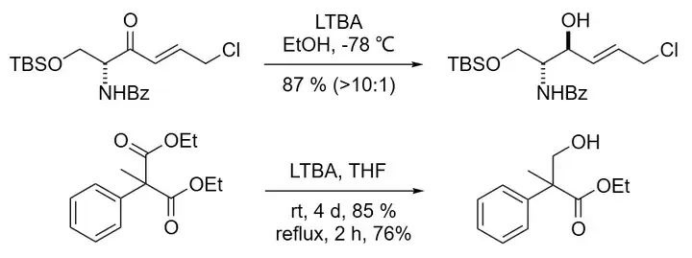
Lewis acids or metal catalysts can enhance the reducing ability of LTBA, enabling the reduction of certain functional groups that are otherwise unreactive under standard conditions.
For example:
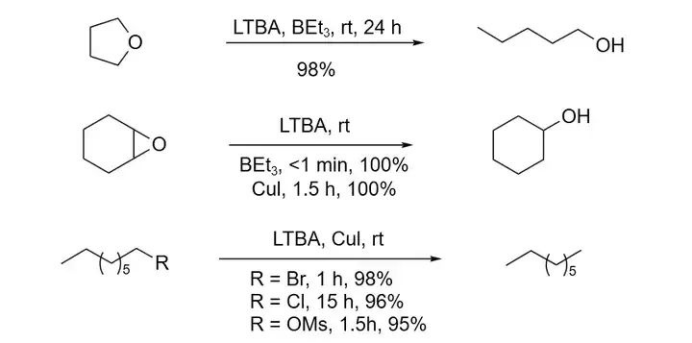
When diborane (B₂H₆) or copper(I) iodide (CuI) is added to the system, LTBA can efficiently reduce ethers and epoxides in high yields.
In the same catalytic system, the reduction rate of alkyl bromides is significantly accelerated.
With prolonged reaction times, even alkyl chlorides can be successfully reduced.
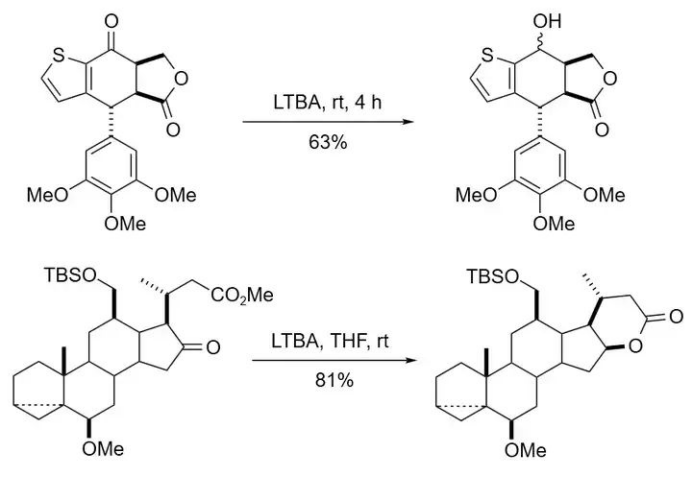
Due to its moderate reducing power, LTBA is most valuable for performing selective reductions.
For example:
In the presence of lactone functional groups, it can selectively reduce aldehydes or ketones while leaving the lactone intact.
This selectivity enables the one-step conversion of δ-keto esters into cyclic lactones.
Guangzhou Lingo Scientific Co — Delivering professional solutions and technical support for the global chemical industry. Our expert team is always ready for your technical inquiries! EMAIL:sales@lingochem.com