[English Name] Lithium Diisopropylamide
[Molecular Weight] 107.15
[CA Registry Number] [4111-54-0]
[Abbreviations and Aliases] LDA
[Structural Formula] i-Pr₂NLi
[Physical Properties] This reagent is soluble in Et₂O, THF, DME, and HMPA but becomes unstable when the temperature rises above 0 °C. However, its solutions in hexane or pentane can remain stable at room temperature for several weeks. Its THF complex is quite stable in cyclohexane and heptane.
[Preparation and Availability] This reagent is a white solid and is commercially available in various solution forms from major international chemical suppliers. In laboratories, it is typically prepared in situ by reacting anhydrous diisopropylamine with n-butyllithium at low temperatures.
[Precautions] The reagent is relatively sensitive to air and moisture, so it is recommended to handle and use it under anhydrous conditions.
Lithium diisopropylamide (LDA) is a widely used sterically hindered, non-nucleophilic strong base in organic synthesis, with a pKa = 35.7 in THF[1]. It is commonly employed to generate carbanions and enolate ions.
[Acidity and Basicity of Common Compounds]
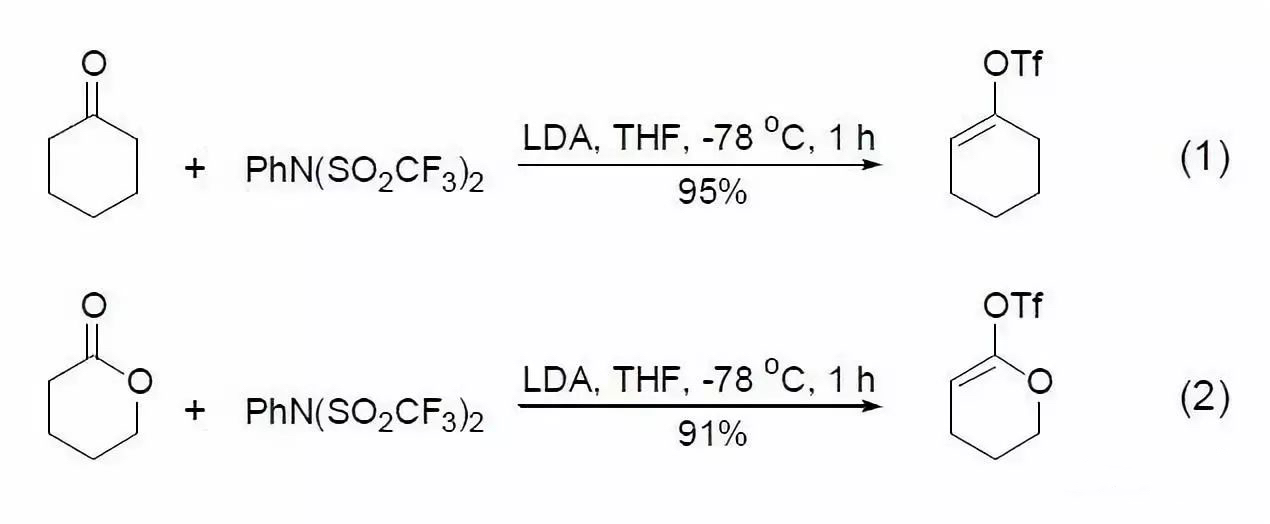
LDA is extensively used in the enolization of aldehydes, ketones, and esters. The resulting enolate ions can be trapped by silicon derivatives or sulfonyl groups to form corresponding silyl ethers or sulfonates. Since the sulfonate group is attached to an sp²-hybridized carbon, it readily undergoes subsequent metal-catalyzed reactions, making this process particularly valuable. The reaction is typically completed within minutes under mild low-temperature conditions and does not significantly affect other functional groups. The yield of the product primarily depends on the reactivity of the trapping reagent, with trifluoromethylsulfonates often yielding satisfactory results (Eq. 1, Eq. 2)[2,3].
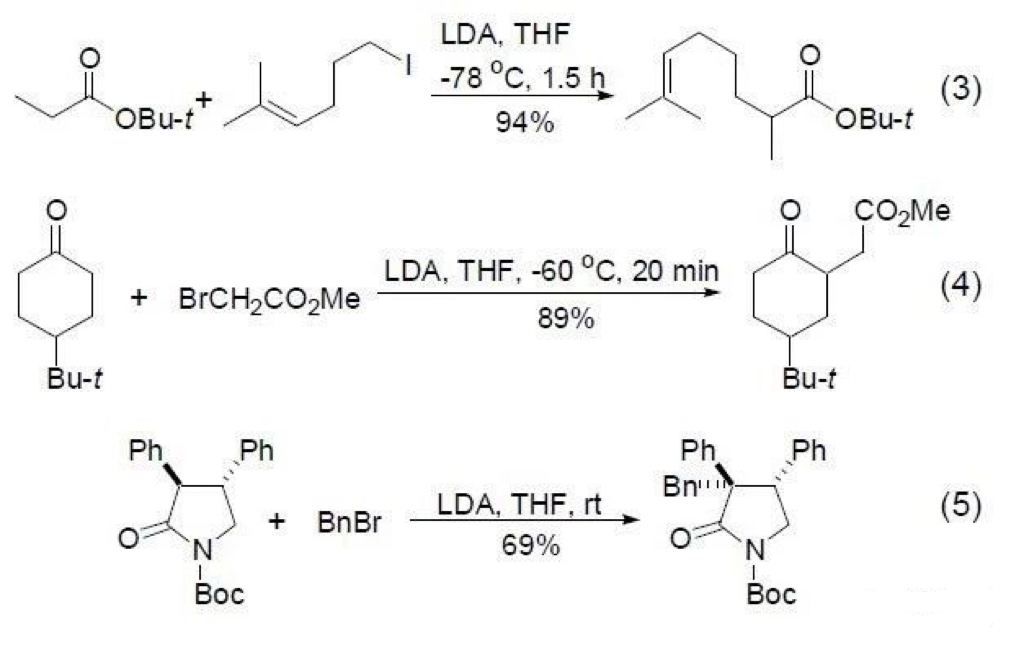
LDA (lithium diisopropylamide) is most directly employed to catalyze the α-alkylation of aldehydes, ketones, esters, and amides [4-10]. Due to the activation of the α-methylene and methine groups by the carbonyl group in these compounds, LDA can deprotonate them at low temperature to generate carbanions. In the presence of various alkylating agents, a wide variety of groups can be conveniently introduced. Because this reagent decomposes above 0 °C, it is sometimes necessary to add an equimolar amount of HMPA (hexamethylphosphoramide) as a cosolvent to enhance the reagent's reactivity and stability. The reaction is typically complete within 1-2 hours at low temperature. Alkene bonds remain completely unaffected under these conditions (Eq 3) [4-6], and ester groups within the alkylating agent are also unaffected (Eq 4) [4-7]. However, reactions involving amides require warming to room temperature (Eq 5) [8].
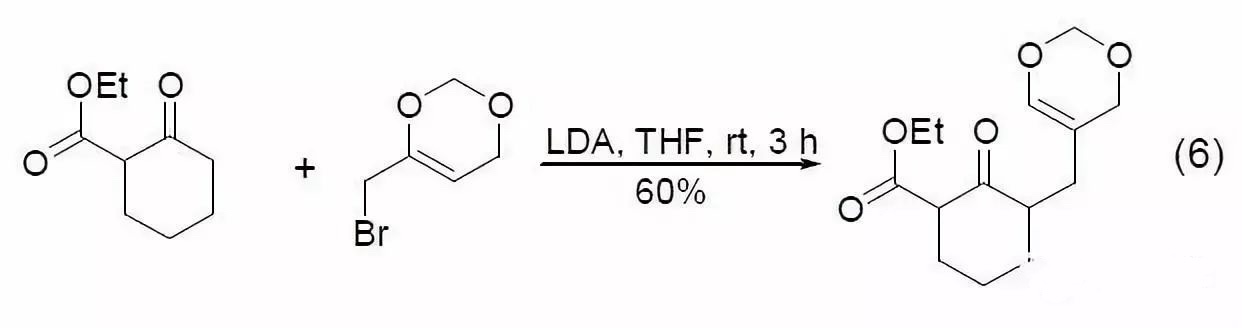
When 1,3-dicarbonyl compounds undergo α-alkylation under the action of LDA, steric hindrance causes alkylation to occur not at the expected more acidic C2 position, but instead at the adjacent α-position. This reaction demonstrates excellent regioselectivity in the case of 1,3-ketoesters (Eq. 6)[11,12].
References
1. Fraser, R. R.; Mansour, T. S. J. Org. Chem., 1984, 49, 3442.
2. Wentworth, A. D.; Wentworth, P.; Mansoor, U. F.; Janda, K.D., Org. Lett., 2000, 2, 477.
3. Kim, D.; Lee, Ju Young; P., Hyun-Ju; T., Khac M. Bioorg. Med. Chem. Lett., 2004, 14, 2099.
4. Bydal, P.; Auger, S.; Poirier, D. Steroids, 2004, 69, 325.
5. Krafft, M. E.; Cheung, Y. Y.; Abboud, K. A. J. Org. Chem., 2001, 66, 7443.
6. Wallace, G.A.; Heathcock, C. H. J. Org. Chem., 2001, 66, 450.
7. Suzuki, H.; Kuroda, C. Tetrahedron, 2003, 59, 3157.
8. Timothy A.; Jang, D. O; Slafer, B. W.; Curtis, M. D.; Beak, P. J. Am. Chem. Soc., 2002, 124, 11689.
9. Sugiyama, H.; Shioiri, T.; Yokokawa, F. Tetrahedron Lett., 2002, 43, 3489.
10. Humphrey, J. M.; Liao, Y.; Ali, A.; Rein, T.; Wong, Y.; Chen, H.; Courtney, A. K.; Martin, S. F. J. Am. Chem. Soc., 2002, 124, 8584.
11. Zhang, W.; Pugh, G. Tetrahedron, 2003, 59, 4237.
12. Greshock, T. J.; Funk, R. L. J. Am. Chem. Soc., 2002, 124, 754.
Guangzhou Lingo Scientific Co — Delivering professional solutions and technical support for the global chemical industry. Our expert team is always ready for your technical inquiries! EMAIL:sales@lingochem.com