In recent years, solid-state batteries (SSBs) have emerged as next-generation energy storage technology beyond traditional lithium-ion batteries due to their high safety and energy density. Sulfide solid electrolytes have attracted significant attention owing to their high ionic conductivity and processability. However, the chemo-mechanical stability of the cathode-electrolyte interface remains a critical challenge. The volume changes of cathode active materials (CAMs) during charging and discharging can lead to interfacial contact loss, formation of a resistive cathode electrolyte interphase (CEI), and trigger oxidative decomposition of sulfide electrolytes, resulting in irreversible capacity loss. Although existing inorganic coatings (e.g., LiNbO₃, LiZrO₃, and LiHfO₂) can partially alleviate these issues, their relatively low ionic conductivity and electronic permeability limit their applicability. Therefore, there is an urgent need to develop multifunctional interfacial layers that combine electrochemical/chemical stability, ionic conductivity, and the ability to buffer volume changes at the CAM/SE (solid electrolyte) interface.
To address these interfacial challenges, researchers haveturned to multifunctional polymer coatings, such as ion-conducting polymers (e.g., PEDOT, LiPAA) and polymeric ionic liquids (PILs). These materials offer chemical stability, ionic conductivity, and flexible buffering capabilities, effectively suppressing electrolyte decomposition and accommodating volume changes. Cationic PILs, such as poly(diallyldimethylammonium) bis(trifluoromethanesulfonyl)imide (PDDATFSI), not only enhance interfacial stability but also mitigate the polysulfide shuttle effect. Experimental results have shown that optimized polymer coatings (e.g., PVBA-TFSI-coated NMC811) significantly reduce interfacial impedance and extend battery cycle life. However, the specific mechanisms of polymer coatings, particularly the influence of lithium salt addition on ion transport and interfacial stability, require further investigation.
Based on this, a research team led by Fokko M. Mulder at Delft University of Technology applied cationic PIL PDDATFSI (with and without LiTFSI) to coat the surface of a nickel-rich cathode NMC82 (LiNi0.82Mn0.07Co0.11O₂), creating two functional coatings—Li-PIL and PIL (Fig. 1)—to elucidate the performance enhancement mechanisms of (Li)PIL coatings in batteries.
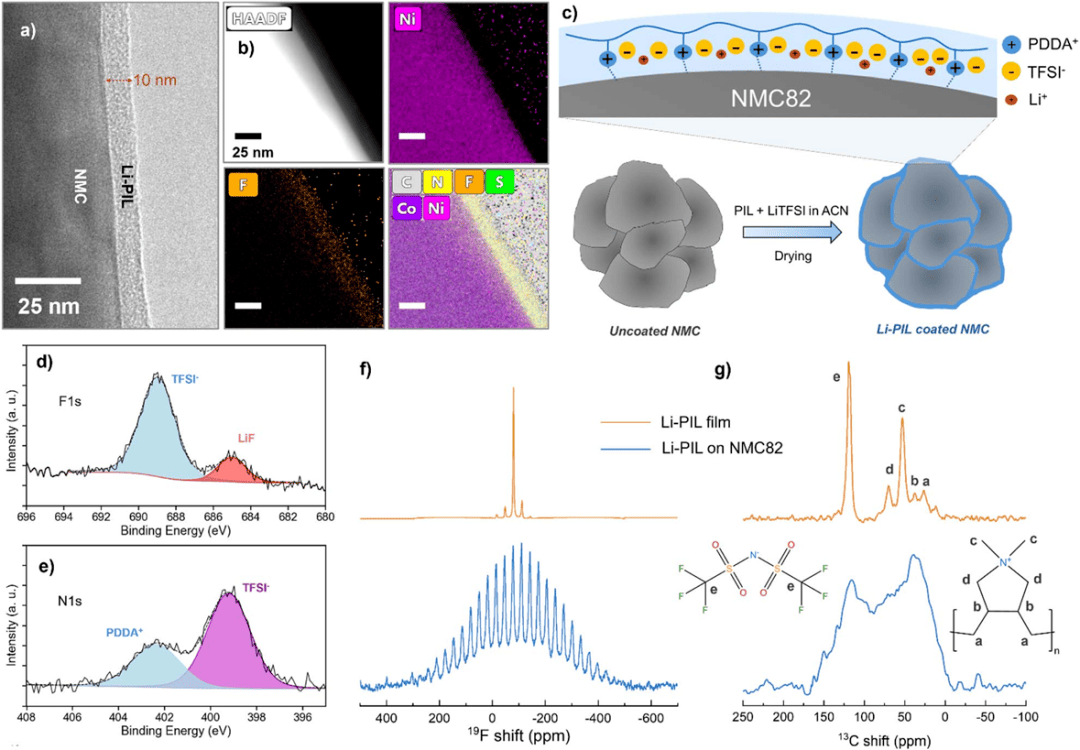
Fig. 1. Structural and chemical characterization of PIL-coated NMC82 materials.
First, the researchers prepared 2 wt% Li-PIL and 1 wt% PIL-coated NMC82 cathode materials and characterized their structural and chemical properties (Fig. 1). TEM analysis revealed that both polymers formed uniform coatings of 5–25 nm, with Li-PIL exhibiting thicker deposition in the interparticle gaps. EDX elemental mapping confirmed the homogeneous distribution of characteristic elements such as F and S on the surface. XPS measurements showed characteristic peaks at 689 eV (TFSI⁻) and 402.5 eV (PDDA⁺), verifying the chemical composition of the coatings. Solid-state NMR detected a broad background signal at 800 ppm in the ¹⁹F spectrum and a broadened TFSI⁻ peak at 80 ppm, indicating nanoscale interactions between the coating and the NMC82 core. XRD confirmed that the coated materials maintained a layered structure with essentially unchanged lattice parameters.
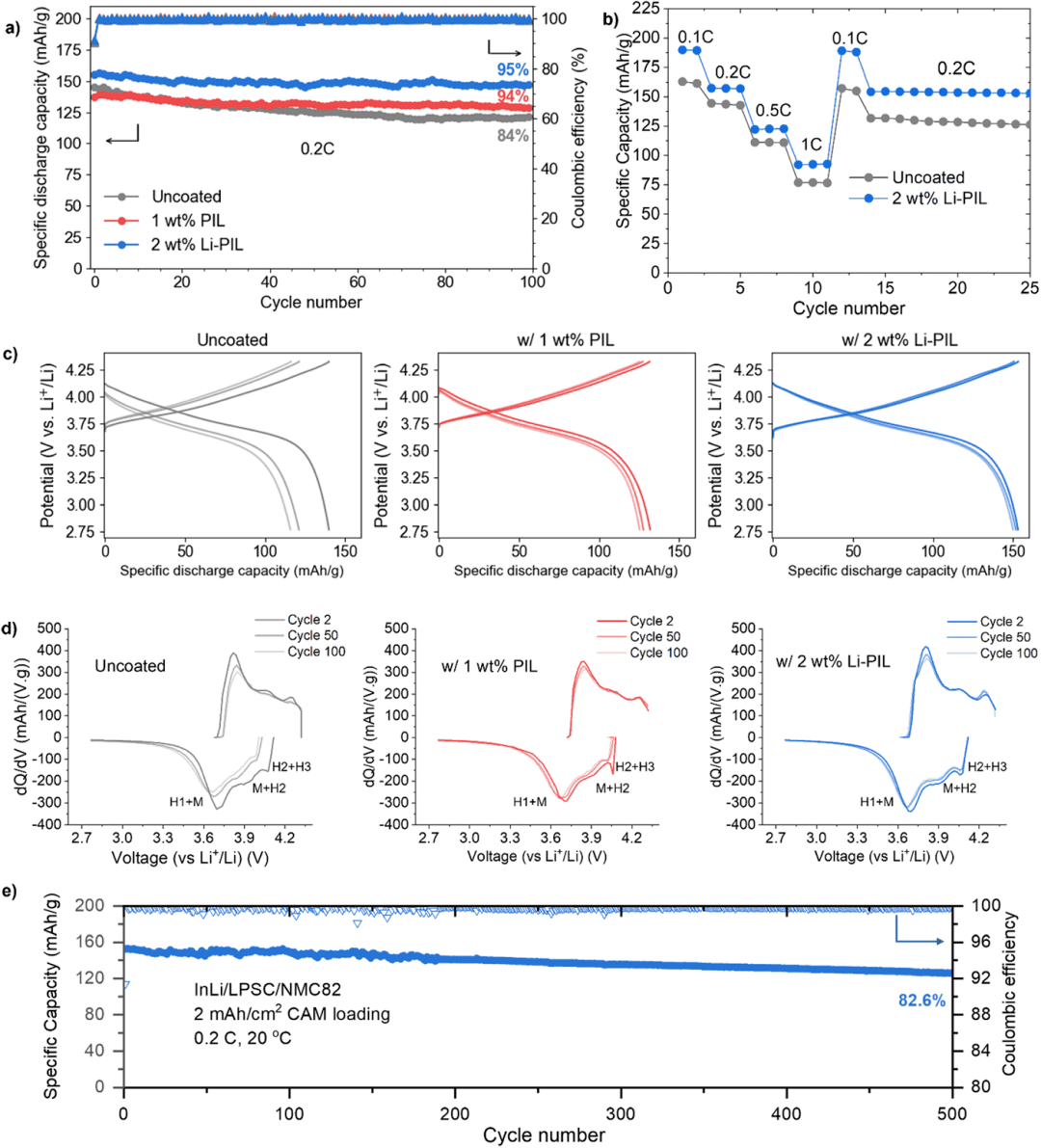
Fig. 2. Comparison of electrochemical performance.
Secondly, the researchers systematically evaluated the impact of (Li)PIL coatings on the performance of solid-state batteries through electrochemical tests (Fig. 2). The Li-PIL-coated NMC82 demonstrated the most superior overall performance: an initial discharge capacity of 155.2 mAh g⁻¹ and a capacity retention of 94% after 100 cycles. Rate capability tests showed that it maintained the highest capacity within the range of 0.1 C to 1 C, with a capacity recovery rate of 98.2%. The dQ/dV curves confirmed that the Li-PIL coating effectively preserved the reversibility of the H2-H3 phase transition, with no observable side reaction peaks even after 100 cycles. Under a condition of 2 mAh cm⁻², the capacity retention remained as high as 82.7% after 500 cycles. Impedance analysis revealed that the Li-PIL coating reduced the interfacial charge transfer resistance by 40% and lowered the activation energy for lithium-ion transport to 0.32 eV.
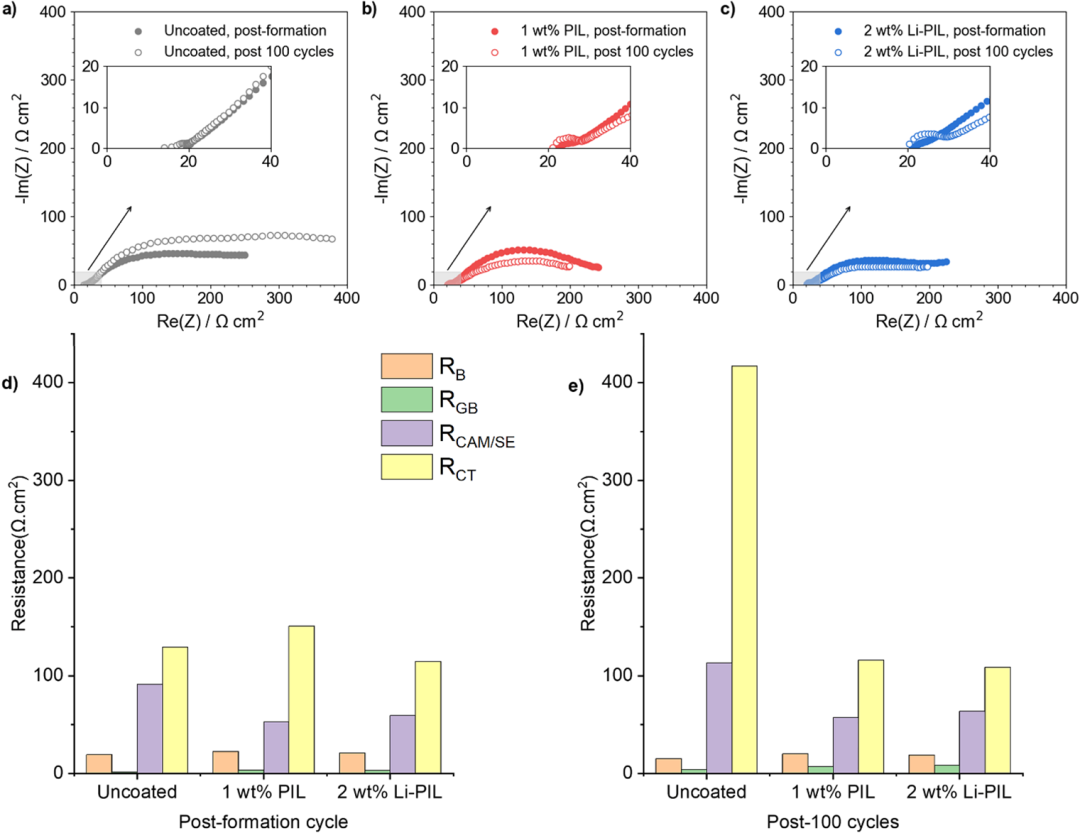
Fig. 3. Electrochemical Impedance Analysis
Furthermore, the researchers analyzed the evolution of interfacial resistance through impedance spectroscopy (Fig. 3). The uncoated NMC82 exhibited the highest initial cathode/electrolyte contact resistance (R<sub>CAM/SE</sub> = 28 Ω cm²), and after 100 cycles, the charge transfer resistance (R<sub>CT</sub>) surged to 4 times its initial value. This was directly correlated with the thickening of the CEI layer and interfacial contact loss caused by the decomposition of Li<sub>6</sub>PS<sub>5</sub>Cl. In contrast, the Li-PIL-coated sample demonstrated superior interfacial stability: the initial R<sub>CT</sub> was reduced by 40%, and the post-cycling increase was minimal (only 1.8 times). Additionally, the activation energy for lithium-ion transport decreased to 0.32 eV. Distribution of Relaxation Times (DRT) analysis confirmed that this coating effectively suppressed interfacial side reactions and maintained stable mechanical contact, thereby ensuring long-term cycling performance.
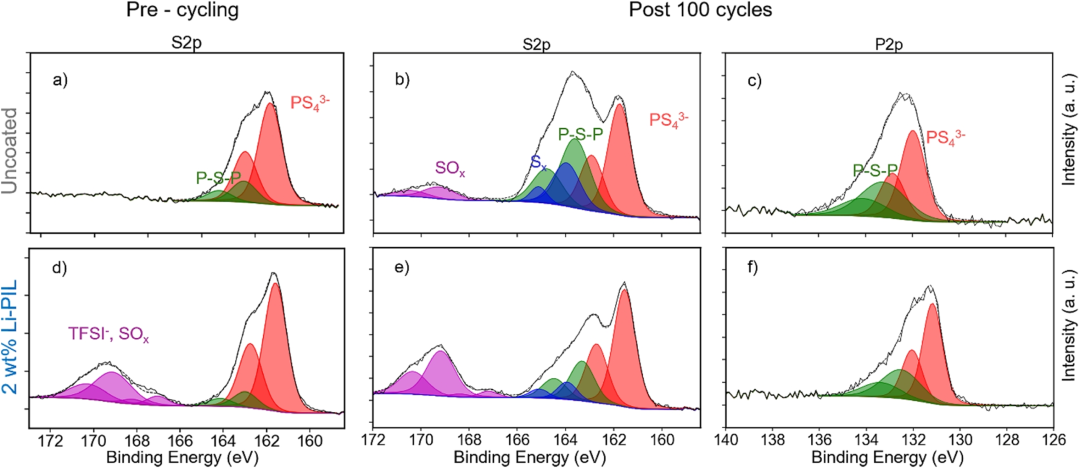
Fig. 4. Electrochemical and Chemical Stability Analysis
Subsequently, the researchers utilized XPS to elucidate the influence of the coating on interfacial chemical stability (Fig. 4). For the uncoated NMC82, significant signals of oxidized species—P–S–P (162.9 eV) and Sₓ (163.9 eV)—were detected in the S 2p spectrum after cycling. The relative content of these species was 2.3 times higher compared to the Li-PIL-coated sample, along with the appearance of a characteristic sulfate (SO₄²⁻, 169.0 eV) peak. Similarly, the P 2p spectrum revealed that the intensity of the P–[S]ₙ–P polysulfide signals in the uncoated sample was 1.8 times higher than in the coated sample, with longer sulfur chains observed. Quantitative analysis demonstrated that the Li-PIL coating reduced the decomposition products of Li₆PS₅Cl by over 60%, while the TFSI⁻ components remained stable before and after cycling.
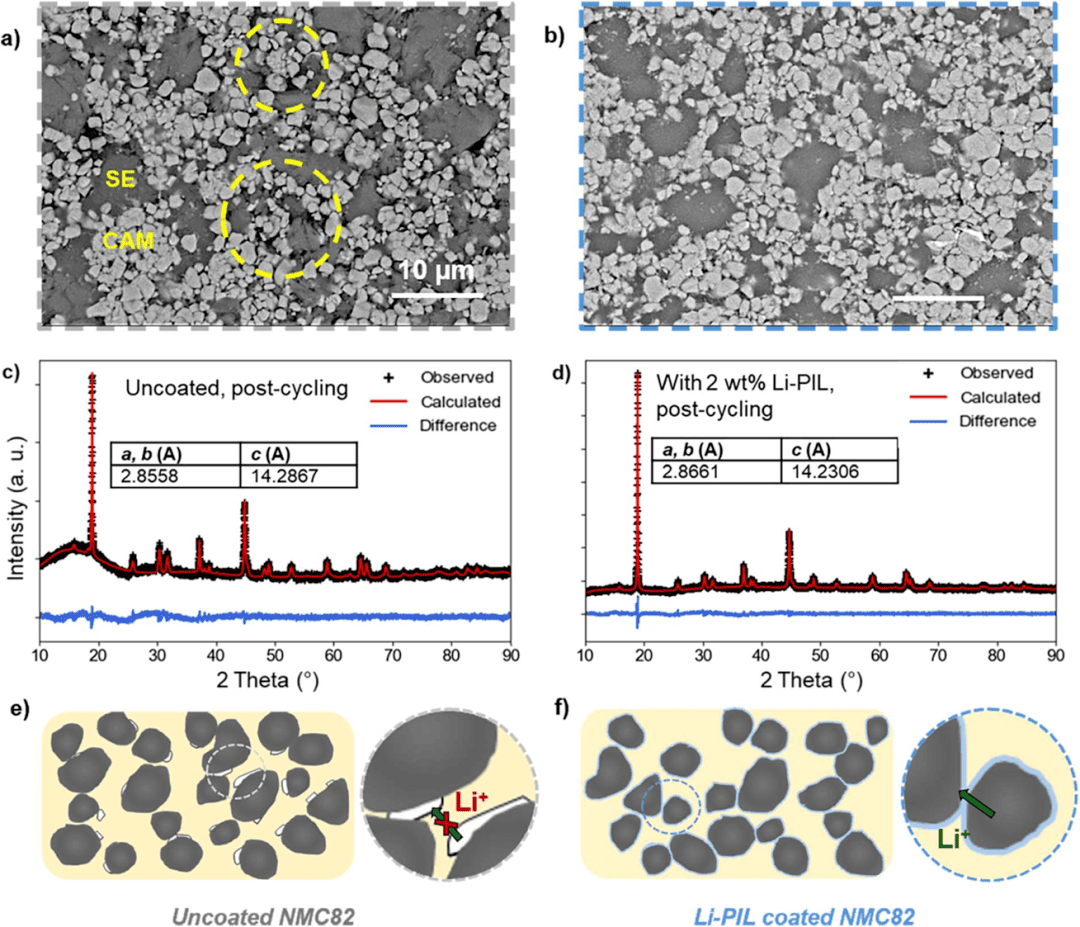
Fig. 5. Analysis of Mechanical and Structural Stability
Finally, the researchers revealed the mechanical buffering effect of the coating through microscopic morphological and structural analyses (Fig. 5). SEM images showed severe interfacial porosity in the uncoated NMC82 after cycling, while the Li-PIL-coated sample maintained intimate CAM/SE contact. The shift and broadening of the XRD (003) peak were significantly reduced, confirming smaller lattice parameter changes in the coated sample. Compression tests demonstrated that Li-PIL exhibited a 37% elastic recovery rate, effectively buffering volume strain through a viscoelastic dissipation mechanism. In summary, this work developed a lithium salt-modified PIL coating technology that successfully addresses the challenges of interfacial contact failure, electrolyte decomposition, and ion transport bottlenecks in nickel-rich cathodes for sulfide-based solid-state batteries, providing an innovative interfacial engineering strategy for developing high-performance solid-state batteries.
Original author(s):Pranav Karanth, Jelle H. Prins, Ajay Gautam, Zhu Cheng, Jef Canals-Riclot, Swapna Ganapathy, Pierfrancesco Ombrini, Alix Ladam, Sebastien Fantini, Marnix Wagemaker, and Fokko M. Mulder
Guangzhou Lingo Scientific Co., Ltd. — Delivering professional solutions and technical support for the global chemical industry. Our expert team is always ready for your technical inquiries! EMAIL:sales@lingochem.com